Synthesis, crystal structures, and spectral properties of double N-alkylated dimethine cyanine dyes and their interactions with biomolecules and living cells†
Abstract
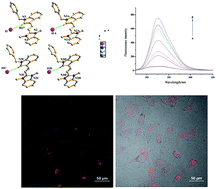
Six new double N-alkylated dimethine cyanine dyes were synthesized and the crystal structures of two of the dyes were analyzed by X-ray diffraction. The investigation of the spectral properties of the dyes in different solvents indicated that the absorption maxima of the dyes decreased with the increase of the basicity of the heterocyclic nucleus, and the increase of the solvent dielectric constants of the protonic solvents and non-protonic solvents. The six dyes all emitted fluorescence, and had a larger Stokes shift in water. The interaction between the dye molecules and the six biological molecules showed that one dye exhibited a larger enhancement of fluorescent quantum yield in the presence of DNA. Investigation of the cytotoxicity and cell staining of the selected dye showed that the dye had virtually no toxicity at the application dose and duration used and that it could stain cytoplasm, suggesting its potential application as a fluorescent reagent with which to observe and analyze the characteristics of living cells.

 Please wait while we load your content...
Please wait while we load your content...