Physicochemical properties of inclusion complexes of sanguinarine with natural cyclodextrins: spectroscopy, calorimetry and NMR studies†
Abstract
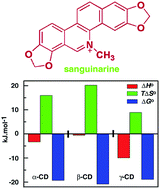
The supramolecular interactions for the formation of inclusion complexes of sanguinarine with α-, β-, and γ-cyclodextrins (CDs) were studied by UV-vis absorbance, fluorescence, circular dichroism and proton nuclear magnetic resonance spectroscopy, and isothermal titration calorimetry. The results revealed that sanguinarine binds with the three natural CDs in 1 : 1 stoichiometry. The binding affinity followed the order β > α > γ-CDs; the affinity to β-CD was the highest compared to the other two CDs apparently due to the perfect cavity size for the inclusion of sanguinarine into the β-CD cavity. The association of sanguinarine with α-, and β-CDs is synergistically driven by a greater entropy contribution to the Gibbs energy of the association, whereas it was favoured by both enthalpy and entropy contributions for the γ-CD. The NMR study indicated that encapsulation of sanguinarine in α-, and β-CDs, involves partial inclusion and to γ-CD it is non-specific and complete inclusion i.e., both ends of sanguinarine, can be included into the CD cavity.

 Please wait while we load your content...
Please wait while we load your content...