Novel triethylamine catalyzed S → O acetyl migration reaction to generate candidate thiols for construction of topological and functional sulfur-containing polymers†
Abstract
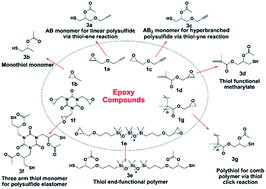
We describe a novel triethylamine catalyzed S → O acetyl migration reaction for yielding thiol compounds under mild conditions through the formation of a transitional 5-membered ring. A series of epoxy compounds have been transformed into their thiol counterparts which could be used for construction of topological and functional sulfur-containing polymers. The one-pot two-step processes including the S → O acetyl migration and the following thiol-click reactions avoided separation of thiol intermediates. Applying these processes on a new-type latent polythiols overcomes crosslinking problem usually met in preparation of multithiol compounds due to the formation of disulfide bonds.

 Please wait while we load your content...
Please wait while we load your content...