Diastereoselective synthesis of cyclopentanone-fused spirooxindoles by N-heterocyclic carbene-catalyzed homoenolate annulation with isatilidenes†
Abstract
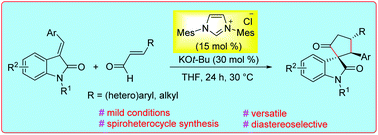
N-Heterocyclic carbene (NHC)-catalyzed formal [3 + 2] annulation of α,β-unsaturated aldehydes with N-substituted isatilidenes resulting in the diastereoselective synthesis of cyclopentanone-fused spirooxindoles is demonstrated. Mechanistically, the reaction proceeds via the generation of homoenolate equivalent intermediates from NHC and enals, which on interception with isatilidenes afford spiro-heterocyclic compounds bearing an all-carbon quaternary spiro-center in moderate to good yields and generally with high diastereoselectivity. Moreover, the functionalization of the spirooxindoles as well as the initial studies on the enantioselective version of this reaction are presented.
- This article is part of the themed collection: Carbenes for Today

 Please wait while we load your content...
Please wait while we load your content...