γ-Lactams and furan bispyrrolidines via iodine mediated cyclisation of homoallylamines†
Abstract
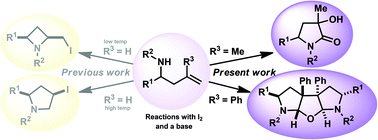
1,3,5-Substituted pyrrolidin-2-ones were synthesised via an iodine mediated cyclisation of 3-methyl substituted homoallylamines in good to excellent yield, as mixtures of diastereoisomers. These were separable and their identity confirmed by techniques including single crystal X-ray diffraction. When 3-phenyl substituted homoallylamines were cyclised intriguing fused tricyclic motifs were obtained as C2-symmetric racemates, whose structures were also confirmed by techniques including single crystal X-ray diffraction analysis.
- This article is part of the themed collection: 2015 Emerging Investigators by OCF

 Please wait while we load your content...
Please wait while we load your content...