Thioether-directed acetoxylation of C(sp2)–H bonds of arenes by palladium catalysis†
Abstract
The palladium-catalyzed acetoxylation of aromatic C(sp2)–H bonds utilizing thioether as the directing group was developed. Both benzyl sulfides and phenethyl sulfides could react with PhI(OAc)2 to afford the site-selectively acetoxylated products in good yields. The directing groups could be further transformed into synthetically useful functional groups or successfully removed.
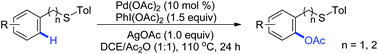

 Please wait while we load your content...
Please wait while we load your content...