Studies on the asymmetric synthesis of pandamarilactonines: an unexpected syn-selective vinylogous Mannich reaction of N-tert-butanesulfinimines†
Abstract
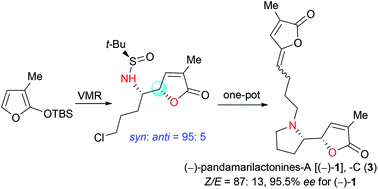
The synthesis of pandamarilactonines in high enantiopurity is challenging due to the configurational instability of the pyrrolidin-2-yl butenolide moiety present in these alkaloids. In an attempted asymmetric synthesis of pandamarilactonine-B (2), an unanticipated syn-diastereoselective (dr = 95 : 5) asymmetric vinylogous Mannich reaction (VMR) between 3-methyl-2-(tert-butyldimethylsilyloxy)furan 9b and the Ellman (RS)-N-tert-butanesulfinimine 10a was observed. The methyl group at C-3 of TBSOF has been shown to play the role of a switch of diastereoselection in the VMR. This work ended with a concise three-pot protecting-group-free total synthesis of (−)-pandamarilactonine-A. The puzzle about the configurational instability of the pyrrolidinyl butenolide skeletons has also been disclosed by controlled experiments and with the help of computation.

 Please wait while we load your content...
Please wait while we load your content...