Conditions for palladium-catalyzed direct arylations of 4-bromo and 4-iodo N-substituted pyrazoles without C–Br or C–I bond cleavage
Abstract
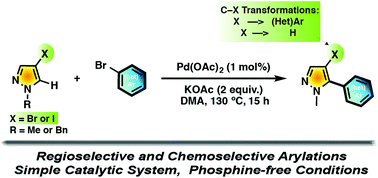
The Pd-catalyzed arylation at the C5 position of N-protected pyrazole derivatives bearing bromo or iodo substituents at the C4 position is described. A simple phosphine-free catalytic system was used, namely, 1 mol% Pd(OAc)2 in DMA in the presence of KOAc as the base. A wide aryl bromide scope as a coupling partner has been coupled with pyrazole derivatives. The reaction was very chemoselective as the C–halogen bonds of the pyrazole units were not involved in the C–H bond arylation process. Some examples demonstrating the synthetic potential of the bromo and iodo pyrazole substituents for chemical transformations are reported.

 Please wait while we load your content...
Please wait while we load your content...