Alkynyl trifluoromethyl selenide synthesis via oxidative trifluoromethylselenolation of terminal alkynes†
Abstract
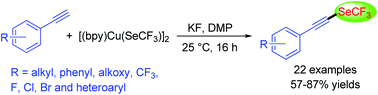
The synthesis of alkynyl trifluoromethyl selenides by Cu-mediated oxidative trifluoromethylselenolation of terminal alkynes is reported. Treatment of [(bpy)Cu(SeCF3)]2 with various terminal alkynes in the presence of an oxidant, Dess–Martin periodinane, led to the formation of trifluoromethylselenolated acetylenes bearing sensitive moieties such as alkoxy, halogens, trifluoromethyl, and heterocyclic groups.

 Please wait while we load your content...
Please wait while we load your content...