Stereoconvergent synthesis of 1-deoxynojirimycin isomers by using the 3 component 4 centred Ugi reaction†
Abstract
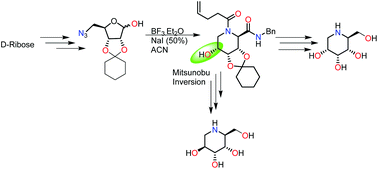
A new reductive cyclization/Ugi multicomponent reaction sequence for the synthesis of 1-deoxyallonojirimycin and 1-deoxyaltronojirimycin has been developed. The method was successfully applied to the azido-hemiacetal derived from commercially available D-ribose. The very selective reagents were used for the synthesis of Ugi bis-amides which were sub sequentially hydrolysed to iminosugars.


 Please wait while we load your content...
Please wait while we load your content...