Deuterative cyclization of sulfanyl 1,6-diynes: complete and monodeuteration of functional groups on heterocycles†‡
Abstract
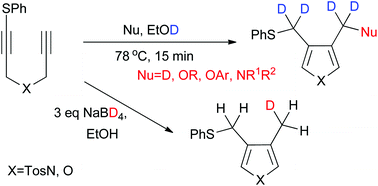
Regioselective H/D exchange reaction of functional groups on heterocycles proceeded via a transition metal-free reductive cyclization of sulfanyl 1,6-diynes using sodium borodeuteride/ethanol-D1. Both alkoxide- and aryloxide-mediated cyclizations and amination–cyclization resulted in the deuteration of functional groups with high deuterium incorporation. Reductive cyclization using sodium borodeuteride/ethanol exclusively afforded the monodeuterated furans and pyrroles in good yields.
- This article is part of the themed collection: Celebrating the 80th Birthday of Professor Ei-ichi Negishi

 Please wait while we load your content...
Please wait while we load your content...