Oxidative C(sp3)–H functionalization of acetonitrile and alkanes with allylic alcohols under metal-free conditions†
Abstract
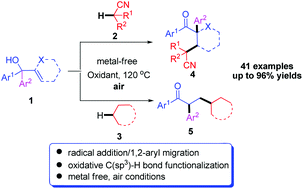
An efficient method for the construction of ketonitriles and α-aryl ketones by tert-butyl peroxybenzoate (TBPB) mediated direct alkylation of acetonitrile and alkanes with α,α-diaryl allylic alcohols has been developed. The reactions involve C(sp3)–H functionalization and radical addition/1,2-aryl migration processes under metal-free conditions.

 Please wait while we load your content...
Please wait while we load your content...