Novel strategies for catalytic asymmetric synthesis of C1-chiral 1,2,3,4-tetrahydroisoquinolines and 3,4-dihydrotetrahydroisoquinolines
Abstract
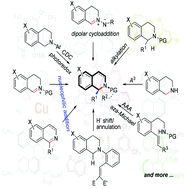
1,2,3,4-Tetrahydroisoquinoline is one of the most important “privileged scaffolds” present in natural products. C1-chiral tetrahydroisoquinolines have exhibited a wide variety of bioactivities and found applications as chiral scaffolds in asymmetric catalysis. This paper summarizes novel catalytic stereoselective strategies that emerged in the last ten years for synthesis of 1,2,3,4-tetrahydroisoquinoline and 3,4-dihydroisoquinoline scaffolds, beyond the traditional Pictet–Spengler and related protocols, as well as their applications in the total synthesis of alkaloid natural products.
- This article is part of the themed collection: 2015 Organic Chemistry Frontiers Review-type Articles

 Please wait while we load your content...
Please wait while we load your content...