Cationic Pd(ii)-catalyzed cyclization of N-tosyl-aniline tethered alkynyl ketones initiated by hydropalladation of alkynes: a facile way to 1,2-dihydro or 1,2,3,4-tetrahydroquinoline derivatives†
Abstract
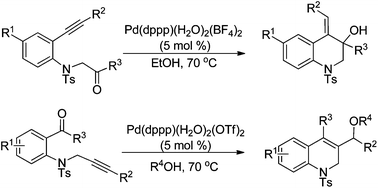
A cationic Pd(II)-catalyzed intramolecular alkyne-carbonyl reductive coupling reaction of N-tosyl-aniline tethered alkynyl ketones under transfer hydrogenation conditions to give hydroquinolines is developed.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2015 and Celebrating the 80th Birthday of Professor Ei-ichi Negishi

 Please wait while we load your content...
Please wait while we load your content...