Improved TTF functionalization of polymers for two-dimensional charge-transfer networks†
Abstract
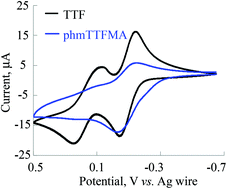
Covalent conjugation of tetrathiafulvalene (TTF) moieties to macromolecular backbones combines unique properties of polymers, such as processability and high functional group density, with the outstanding redox properties of TTF to expand applications in organic electronics, chemical sensors, molecular switches, and nonlinear optical materials among others. Herein, we report the synthesis of a polymethacrylate backbone with 4-(hydroxymethyl)TTF (hmTTF) as the pendant group using post-polymerization modification of poly(N-hydroxysuccinimide methacrylate) to obtain the product polymer with a wide range of molecular weights (18 ≤ Mn ≤ 126 kDa) and up to ca. 70 mol% (76 wt%) TTF. The electrochemical and electronic properties of phmTTFMA are analogous to that of monomeric TTF. Film-forming phmTTFMA-based charge-transfer complexes (CTCs) with various electron acceptors including iodine, TCNQ, and chloranil showed an isotropic electrical conductivity. The magnitude of the conductivities of phmTTFMA-based CTCs is primarily affected by the electron affinity of the electron acceptors. The morphology of the amorphous phmTTFMA is strongly dependent on its electronic interactions with small-molecule electron acceptors. For example, a lamellar-like structure is observed when phmTTFMA is mixed with chloranil.

 Please wait while we load your content...
Please wait while we load your content...