Nitric oxide-releasing polymeric furoxan conjugates†
Abstract
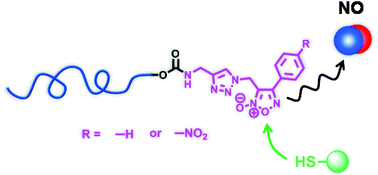
Furoxans, or 1,2,5-oxadiazole-N-oxides, are a class of heterocyclic compounds that release nitric oxide (NO), a gaseous signaling mediator in the human body, by the reaction with thiol-containing molecules. Here, we prepared polymeric furoxan conjugates that may solve the problems associated with the therapeutic use of low molecular weight furoxans. The conjugates were prepared by the copper-catalyzed Huisgen cycloaddition of the corresponding azide-functionalized furoxan derivatives onto poly(ethylene glycol) (PEG) with an alkyne end group. The conjugates released NO in response to cysteine and glutathione. The conjugates also released NO in the presence of the cell lysate of murine macrophages but not in fetal bovine serum. It was found by infrared and 1H NMR spectroscopy that the furoxans undergo decomposition in physiological buffer, which can be slowed down by the conjugation of furoxans to PEG. Furthermore, the PEG–furoxan conjugates released NO and enhanced the anti-proliferative effect of ibuprofen in HT-29 colon cancer cells.

 Please wait while we load your content...
Please wait while we load your content...