The construction of supramolecular polymers through anion bridging: from frustrated hydrogen-bonding networks to well-ordered linear arrays†
Abstract
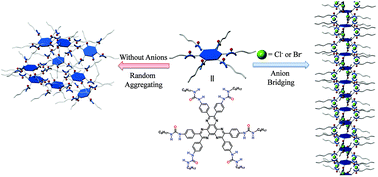
Although it is not difficult to control the direction of a single noncovalent bond, to precisely control the orientation of noncovalent bonding arrays, which is a precondition for the construction of complicated supramolecular systems, is still a great challenge. In this work, we demonstrate the construction of one-dimensional (1D) supramolecular polymers by converting random hydrogen-bonding networks into well-ordered linear hydrogen-bonding arrays through an anion-bridging strategy. The supramolecular polymers were fabricated through the co-assembly of a propeller-like hexaazatriphenylene (HAT) derivative peripherally bearing six urea units with Cl− or Br− ions, driven by the formation of N–H⋯X (X = Cl−, Br−) hydrogen bonds between urea NH and halogen anions, a type of hydrogen bond that has been extensively used for selectively recognizing anionic species but remained unexplored to fabricate supramolecular polymers. These supramolecular polymers exhibited extremely low critical polymerization concentrations and were found to show response toward different stimuli including polarity of solvent, temperature, and metal ions.

 Please wait while we load your content...
Please wait while we load your content...