Effects of replacing thiophene with 5,5-dimethylcyclopentadiene in alternating poly(phenylene), poly(3-hexylthiophene), and poly(fluorene) copolymer derivatives†
Abstract
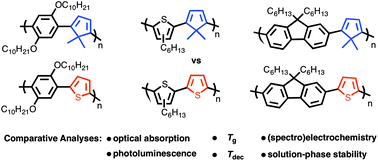
Poly(phenylene), poly(3-hexylthiophene), and poly(fluorene)-based copolymers bearing alternating thiophene (PPT, P3HTT, and PFT) and 5,5-dimethylcyclopentadiene co-repeat units (PPCp, P3HTCp, PFCp) were examined to establish how the identity of the latter manipulates the optical absorption, photoluminescence, thermal properties, (spectro)electrochemistry, and atmospheric stability of these systems. The results of our investigation show that the 4π electron diene moiety reduces the optical band gap of the poly(fluorene) and poly(3-hexylthiophene)-based copolymers when compared against their all-aromatic congeners while having the opposite effect on the poly(phenylene) class. Fluorescence studies reveal that the emission maxima λem of the diene systems are lower in energy among the three copolymer sets, however, quantum yields ΦF are also lower indicating that the diene-moiety compromises photoluminescence. Regarding thermal properties, the identity of the co-repeat unit (i.e. diene vs. thiophene) was found to have a negligible effect on copolymer glass-transition temperature Tg, however, the polyaromatics exhibit higher thermal stability irrespective of copolymer class. Cyclic voltammetry data show that the onset of oxidation (Eonset) decreases in the order of poly(fluorene), poly(phenylene), and poly(3-hexylthiophene) derivatives suggesting that the aromatic co-repeat units play a critical role in establishing the HOMO energy. Moreover, when copolymers within each class are compared, the diene-containing systems always exhibit a lower Eonset indicating that the diene moiety increases the HOMO energy when used in lieu of thiophene. Solution-phase stability studies under ambient atmospheric conditions show the poly(3-hexylthiophene)s to be the most stable class of copolymers followed by the poly(phenylene)s and poly(fluorene)s respectively. In addition to the comparative analyses described herein, three model configurational triads of the poly(3-hexylthiophene) derivative P3HTCp were characterized to allow for the unambiguous assignment of copolymer regiochemistry that was determined to be regiorandom. Finally, a polymer light-emitting diode (PLED) was constructed using PFCp as the emissive layer as a proof-of-principle that diene-containing copolymers can be used as active materials in opto-electronic devices.

 Please wait while we load your content...
Please wait while we load your content...