Effect of fluorine substitution on the photovoltaic performance of poly(thiophene-quinoxaline) copolymers†
Abstract
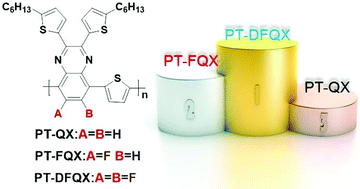
In order to investigate the effects of fluorine atoms on the photovoltaic performance, three 2-D D–A conjugated copolymers, namely PT-QX (0F), PT-FQX (1F) and PT-DFQX (2F), were designed and synthesized using alkylthienyl substituted quinoxaline with different numbers of F substituents as the acceptor unit and thiophene as the donor unit. The physicochemical and photovoltaic properties were comparatively studied in detail. The results demonstrate that the highest occupied molecular orbital (HOMO) energy levels are gradually lowered from −5.10 eV to −5.18 eV and then to −5.33 eV for PT-QX (0F), PT-FQX (1F) and PT-DFQX (2F), respectively, while the lowest occupied molecular orbital (LUMO) energy levels nearly remain constant with the increase of F substituents. Introducing F on the polymer backbone widens the energy bandgap and makes the absorption peaks of the polymers blue-shifted. The highest power conversion efficiencies of bulk heterojuncton polymer solar cells increased with the increase of F substituents from 2.82% for PT-QX (0F) to 4.14% for PT-FQX (1F) to 5.19% for PT-DFQX (2F) thanks to the enhanced Voc and Jsc. The enhanced Voc and Jsc can be mainly ascribed to the lower HOMO energy levels and moderate hole mobility of the fluorinated polymers, as well as the better morphology and preferential orientation of the face-on structure of the blend films of the fluorinated polymer donor with a PC71BM acceptor.

 Please wait while we load your content...
Please wait while we load your content...