Preferential chiral solvation induced supramolecular chirality in optically inactive star Azo polymers: photocontrollability, chiral amplification and topological effects†
Abstract
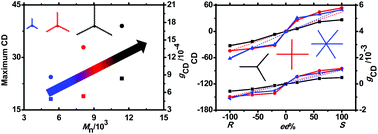
A series of star-shaped azobenzene (Azo)-containing side chain polymers with 3, 4 and 6 arms were synthesized by the atom transfer radical polymerization technique initiated by multifunctional initiators. The intense bisignated circular dichroism (CD) signals in the UV–vis region were observed when these star polymers aggregated in a dichloroethane/limonene mixed solvent, indicating the successful chirality transfer from limonene molecules to these star Azo polymers. The chirality originates from the preferential supramolecular chirality of well-organized optically inactive side-chain Azo units. The supramolecular chirality is closely related to the volume fraction of cosolvents, the enantiopurity (ee) of limonene, molecular weight and arm numbers of star Azo polymers. Interestingly, the maximum values of CD and gCD of the polymer aggregates increase with molecular weight in the case of 3-armed Azo polymers, and increase simultaneously with arm numbers of the polymers having similar repeating units under each optimized experimental condition. Surprisingly, the first chiral amplification behaviours were observed in the 4- and 6-armed Azo polymers, but absent in linear and 3-armed counterparts. The supramolecular chirality can be destroyed by trans–cis photoisomerization and recovered by thermal cis–trans isomerization of Azo units. The current research will provide further insight into the mechanism study and production of effective chiroptical materials in chiral solvation induced supramolecular chirality of the achiral polymers.

 Please wait while we load your content...
Please wait while we load your content...