Design of thiol- and light-sensitive degradable hydrogels using Michael-type addition reactions†
Abstract
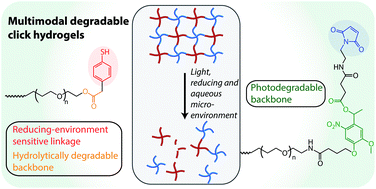
Injectable depots that respond to exogenous and endogenous stimuli present an attractive strategy for tunable, patient-specific drug delivery. Here, the design of injectable and multimodal degradable hydrogels that respond to externally applied light and physiological stimuli, specifically aqueous and reducing microenvironments, is reported. Rapid hydrogel formation was achieved using a thiol-maleimide click reaction between multifunctional poly(ethylene glycol) macromers. Hydrogel degradation kinetics in response to externally applied cytocompatible light, reducing conditions, and hydrolysis were characterized, and degradation of the gel was controlled over multiple time scales from seconds to days. Further, tailored release of an encapsulated model cargo, fluorescent nanobeads, was demonstrated.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...