Towards sugar-derived polyamides as environmentally friendly materials†
Abstract
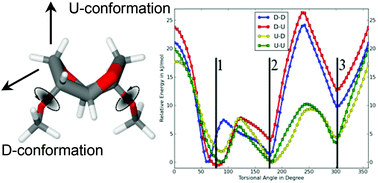
As part of our ongoing study investigating isohexide-based polyamides, we have synthesized isosorbide(bis(propan-1-amine)) (DAPIS) and studied its reactivity in the polymerization towards fully biobased polyamides. Polycondensation of nylon salts with various contributions of DAPIS afforded a family of homo- and copolyamides, which were characterized using complementary spectroscopic techniques. The chemical structure of the materials was determined by FT-IR, 1D and 2D liquid-state NMR spectroscopy, whilst the supramolecular arrangement, conformational changes upon heating, and molecular mobility of the polymers were investigated by solid-state 13C{1H} Cross-Polarization/Magic-Angle Spinning (CP/MAS) NMR and 13C{1H} Insensitive Nuclei Enhanced by Polarization Transfer (INEPT) experiments. The abundance of the different DAPIS conformers was determined by DFT-D computational methods. The thermal properties of the polyamides were tested for polymers with different amounts of isohexide units in the backbone by DSC and TGA, demonstrating that the increasing amounts of isohexide diamines efficiently decrease their melting points and slightly decrease their thermal stability. The relaxation processes of the isohexide-derived polyamides were studied by DMTA.


 Please wait while we load your content...
Please wait while we load your content...