From nanospheres to micelles: simple control of PCL-g-PEG copolymers’ amphiphilicity through thiol–yne photografting†
Abstract
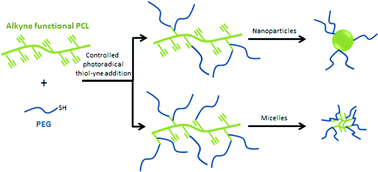
A simple method for the synthesis of a family of poly(ε-caprolactone)-g-polyethylene glycol (PCL-g-PEG) copolymers of controlled amphiphilicity and their use in generating nanospheres or micelles are reported. PCL-g-PEGs with various compositions are prepared from a single strategy relying on a combination of post-polymerization modification and subsequent thiol–yne photografting. Alkyne-functional PCL (PCL-yne) was first obtained by anionic activation and reaction with propargyl bromide to yield PCL-yne with 8% alkyne groups. PEG-thiol is then reacted on PCL-yne under UV activation to yield the targeted graft copolymer by thiol–yne photoaddition. The advantage of the approach is that control over the composition is easily achieved, yielding amphiphilic graft copolymers with ethylene glycol/caprolactone (EG/CL) ratios ranging from 0.1 to 1.3. Starting from this single strategy, it was therefore possible to obtain nanospheres (DH ∼ 55 nm) or micelles (DH ∼ 30 nm) by copolymer self-assembly depending on the EG/CL ratio. The potential of PCL-g-PEG micelles as drug carriers was finally evaluated with curcumin that was efficiently encapsulated, protected and released over 80 days. Interestingly it was found that drug encapsulation efficiency and drug loading were higher for PCL-g-PEG copolymers compared to block PCL-b-PEG.

 Please wait while we load your content...
Please wait while we load your content...