Helical folding of an arylamide polymer in water and organic solvents of varying polarity†
Abstract
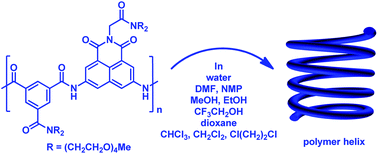
An amphiphilic aromatic amide polymer has been prepared from the condensation of 1,8-naphthalimide-3,6-diamine and isophthalic acid precursors, both of which bear tetraethylene glycol chains. Fluorescence and UV-vis experiments for the polymer and mono-, tri-, penta-, and heptameric control compounds indicate that the polymer can form a helical hollow foldamer in both water and organic solvents of high and low polarity, including N,N-dimethylformamide, methanol, chloroform and dichloromethane. Fluorescence experiments in binary solvents reveal that in benign chloroform or dichloromethane of low polarity, across-layer intramolecular hydrogen bonding is the main driving force for the formation of the helical conformation. In highly polar water and organic solvents like methanol, the solvophobicity is the main driving force. Fluorescence experiments for the corresponding N-methylated polymer indicate that N-methylation reduces the folding propensity of the polymer considerably, but in polar solvents such as methanol, the polymer can still form the helical conformation.

 Please wait while we load your content...
Please wait while we load your content...