Thiol-reactive functional poly(meth)acrylates: multicomponent monomer synthesis, RAFT (co)polymerization and highly efficient thiol–para-fluoro postpolymerization modification†
Abstract
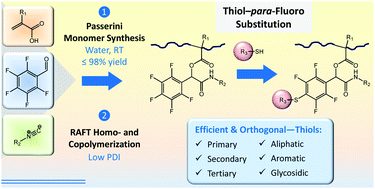
A novel class of thiol-reactive (meth)acrylate monomers and the quantitative postpolymerization modification of their RAFT-made (co)polymers with aromatic, glycosidic, and aliphatic thiols are presented. A set of 6 different N-functional 2-(meth)acryloyloxy-2-(pentafluorophenyl)acetamide monomers bearing pentafluorophenyl groups was prepared by a Passerini three-component reaction of (meth)acrylic acid, 2,3,4,5,6-pentafluorobenzaldehyde, and various isocyanides in water in up to near-quantitative isolated yields. RAFT polymerization was used to produce well-defined homopolymers and copolymers with methyl methacrylate, tert-butyl methacrylate, poly(ethylene glycol) methyl ether (meth)acrylate, and pentafluorophenyl acrylate, with low polydispersity indices of generally ĐM ≤ 1.23. In the presence of base, (co)polymers underwent selective para-fluoro substitution reactions with thiols in the absence of any side reactions observable by 1H and 19F NMR spectroscopy and size exclusion chromatography. The selection of employed thiols included various alkanethiols, a thiolated glucose derivative, mercaptopropionic acid, L-cysteine and the drug captopril. 19F NMR kinetic measurements indicated quantitative thiol–para-fluoro substitutions after <3–80 min at 25–45 °C using 1–1.1 equiv. of thiol, depending on the reactivity of the employed thiol (aromatic, glycosidic > primary aliphatic > secondary aliphatic > tertiary aliphatic) and the choice of a suitable base (triethylamine or 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)). The versatility of thiol-reactive (meth)acrylate species is demonstrated by the examples of a thermoresponsive copolymer showing a thiol-sensitive lower critical solution temperature (LCST) and the selective sequential modification with thiols and amines of a doubly reactive copolymer containing activated pentafluorophenyl esters.

 Please wait while we load your content...
Please wait while we load your content...