A pH-responsive amphiphilic supramolecular graft copolymer constructed by crown ether based molecular recognition†
Abstract
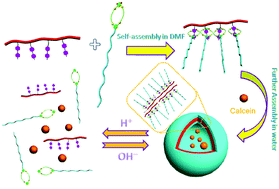
Graft polymers always play a significant role in polymer chemistry as they are preferred materials for a large variety of applications, such as drug/gene delivery systems, hybrid and biomedical materials, soft rubbers, and opto-electronic materials. Compared with traditional graft copolymers, supramolecular graft copolymers whose main chains and side chains are connected by non-covalent forces (e.g. hydrogen bonding, metal-coordination, host–guest interactions, etc.), could be easily obtained in a facile and dynamic manner instead of tedious and time-consuming synthesis. Herein, based on the BMP32C10/paraquat molecular recognition in water, we have successfully prepared an amphiphilic supramolecular graft copolymer by synthesizing poly(styrene-co-paraquat) 1 as the hydrophobic main chain and modified poly(ethylene oxide) 2 as the hydrophilic side chains. The self-assembly behaviour of the amphiphilic supramolecular graft copolymer in water was investigated by DLS, TEM and SEM. As confirmed, it could self-assemble into bilayer vesicles in water, which would be destroyed with the treatment of HCl. Therefore, the vesicles were further used in the pH controlled release of water-soluble molecules.

 Please wait while we load your content...
Please wait while we load your content...