The role of the non-covalent β-ionone-ring binding site in rhodopsin: historical and physiological perspective†
Abstract
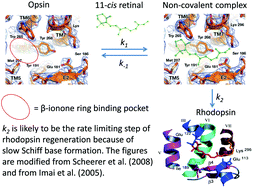
Bleached rhodopsin regenerates by way of the Schiff base formation between the 11-cis retinal and opsin. Recovery of human vision from light adapted states follows biphasic kinetics and each adaptive phase is assigned to two distinct classes of visual pigments in cones and rods, respectively, suggesting that the speed of Schiff base formation differs between iodopsin and rhodopsin. Matsumoto and Yoshizawa predicted the existence of a β-ionone ring-binding site in rhodopsin, which has been proven by structural studies. They postulated that rhodopsin regeneration starts with a non-covalent binding of the β-ionone ring moiety of 11-cis-retinal, followed by the Schiff base formation. Recent physiological investigation revealed that non-covalent occupation of the β-ionone ring binding site transiently activates the visual transduction cascade in the dark. In order to understand the role of non-covalent binding of 11-cis-retinal to opsin during regeneration, we studied the kinetics of rhodopsin regeneration from opsin and 11-cis-retinal and found that the Schiff base formation is accelerated ∼107 times compared to that between retinal and free amine. According to Cordes and Jencks, Schiff base formation in solution exhibits a bell-shaped pH dependence. However, we discovered that the rhodopsin formation is independent of pH over a wide pH range, suggesting that aqueous solvents do not have access to the Schiff base milieu during its formation. According to Hecht et al. the regeneration of iodopsin must be significantly faster than that of rhodopsin. Does this suggest that the Schiff base formation in iodopsin is favored due to its structural architecture? The iodopsin structure once solved would answer such a question as how molecular fine-tuning of retinal proteins realizes their dark adaptive functions. In contrast, bacteriorhodopsin does not require occupancy of a distinct β-ionone ring-binding site, enabling an aldehyde without the cyclohexene ring to form a pigment. Studies of regeneration reaction of other retinal proteins, which are scarcely available, would clarify the molecular structure–phenotype relationships and their physiological roles.
- This article is part of the themed collection: 16th International Conference on Retinal Proteins

 Please wait while we load your content...
Please wait while we load your content...