Producing a dual-fluorescent molecule by tuning the energetics of excited-state intramolecular proton transfer†
Abstract
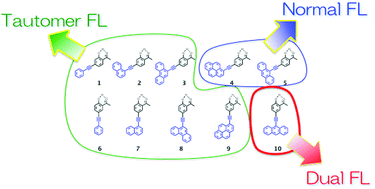
We report herein the selective preparation of normal, tautomeric, and dual-fluorescent molecules with a common ESIPT core. 2′-Hydroxyacetophenone (OHAP) is known as a typical molecule that undergoes excited-state intramolecular hydrogen transfer (ESIPT) to display fluorescence emission from the excited state of the tautomer. In this study, a series of ten OHAP-cored fluorescent molecules were prepared and their excited state properties have been explored. The bathochromic shift of the π–π* absorption band with π-extensions of substituents of these molecules indicates that the excitation energy of the normal form of the OHAP unit was reduced due to the substituents, while the energy of the excited tautomer appeared to be independent of the π-extension of the substituents. When pyrene or anthracene was connected at the end (molecules 4 and 5), only normal fluorescence appeared, and the tautomer fluorescence disappeared. An anthracene derivative (molecule 10) displayed dual fluorescence, indicating that the normal and the tautomer excited states were energetically “balanced”. A fluorescence lifetime analysis revealed the ESIPT reaction rate of 10 to be much slower than those of other derivatives and that the normal and tautomer forms were in equilibrium in the excited state.

 Please wait while we load your content...
Please wait while we load your content...