A search for radical intermediates in the photocycle of LOV domains†
Abstract
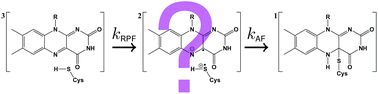
LOV domains are the light sensitive parts of phototropins and many other light-activated enzymes that regulate the response to blue light in plants and algae as well as some fungi and bacteria. Unlike all other biological photoreceptors known so far, the photocycle of LOV domains involves the excited triplet state of the chromophore. This chromophore is flavin mononucleotide (FMN) which forms a covalent adduct with a cysteine residue in the signaling state. Since the formation of this adduct from the triplet state involves breaking and forming of two bonds as well as a change from the triplet to the singlet spin state, various intermediates have been proposed, e.g. a protonated triplet state 3FMNH+, the radical anion 2FMN˙−, or the neutral semiquinone radical 2FMNH˙. We performed an extensive search for these intermediates by two-dimensional transient absorption (2D-TA) with a streak camera. However, no transient with a rate constant between the decay of fluorescence and the decay of the triplet state could be detected. Analysis of the decay associated difference spectra results in quantum yields for the formation of the adduct from the triplet of ΦA(LOV1) ≈ 0.75 and ΦA(LOV2) ≈ 0.80. This is lower than the values ΦA(LOV1) ≈ 0.95 and ΦA(LOV2) ≈ 0.99 calculated from the rate constants, giving indirect evidence of an intermediate that reacts either to form the adduct or to decay back to the ground state. Since there is no measurable delay between the decay of the triplet and the formation of the adduct, we conclude that this intermediate reacts much faster than it is formed. The LOV1-C57S mutant shows a weak and slowly decaying (τ > 100 μs) transient whose decay associated spectrum has bands at 375 and 500 nm, with a shoulder at 400 nm. This transient is insensitive to the pH change in the range 6.5–10.0 but increases on addition of β-mercaptoethanol as the reducing agent. We assign this intermediate to the radical anion which is protected from protonation by the protein. We propose that the adduct is formed via the same intermediate by combination of the radical ion pair.
- This article is part of the themed collection: Photofunction Proteins

 Please wait while we load your content...
Please wait while we load your content...