Domino reactions of diazodicarbonyl compounds with α,β-unsaturated δ-amino esters: a convenient way towards 2-oxopiperidines, dihydropyridinones and isoquinolinediones†
Abstract
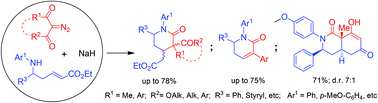
Thermal decomposition of a series of diazodicarbonyl compounds in the presence of α,β-unsaturated δ-amino esters and sodium hydride gives rise to a variety of nitrogenous heterocycles. The direction of these processes is highly dependent on the structure of the initial reagents giving rise to the formation of multi-functionalized 2-oxopiperidines, 5,6-dihydropyridin-2(1H)-ones or tetrahydroisoquinoline-1,6(2H,8aH)-diones. The reactions occur in domino processes involving the Wolff rearrangement of diazocarbonyl compounds, NaH-catalyzed anti-stereoselective intramolecular Michael addition to the α,β-unsaturated system of amino esters, and in some cases also cycloelimination and intramolecular Claisen condensation of the initial products formed.

 Please wait while we load your content...
Please wait while we load your content...