Contrasting anion recognition behaviour exhibited by halogen and hydrogen bonding rotaxane hosts†
Abstract
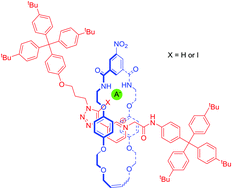
A rotaxane host system containing a novel halogen bonding (XB) 5-iodo-1,2,3-triazole functionalised pyridinium motif, within its axle component, has been prepared via a ring closing metathesis reaction, using chloride as a template. Proton NMR titration experiments, in competitive 1 : 1 CDCl3–CD3OD solvent media, showed the XB rotaxane selectively bound halides over larger, more basic oxoanions. An all hydrogen bonding proto-triazole containing rotaxane analogue was also prepared, which in stark contrast demonstrated a reversal in the anion selectivity trend, with a preference for dihydrogen phosphate over the halides which is unprecedented for an interlocked host system.
- This article is part of the themed collection: In celebration of Paul Beer’s 60th Birthday

 Please wait while we load your content...
Please wait while we load your content...