Expedient synthesis of tetrasubstituted pyrroles via a copper-catalyzed cascade inter-/intramolecular cyclization of 1,3-enynes carry a nitro group with amines†
Abstract
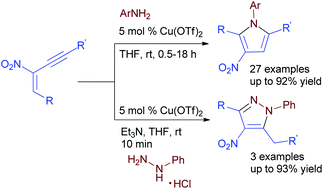
Various tetrasubstituted pyrroles/pyrazoles have been prepared from nitro-substituted 1,3-enynes with aromatic amines/hydrazines via a copper-catalyzed cascade aza-Michael addition, cyclization and aromatization at room temperature. This protocol is also effective for the synthesis of tetrasubstituted pyrazoles in high yields.

 Please wait while we load your content...
Please wait while we load your content...