Pd-catalyzed Heck-conjoined amidation and concomitant chemoselective Michael-addition: an efficient tandem approach to highly functionalized tetrahydroquinazolines from o-haloanilines†
Abstract
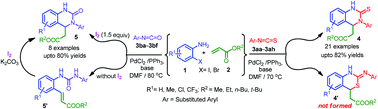
An efficient palladium-catalyzed tandem approach for the synthesis of highly functionalized tetrahydroquinazolines 4a–q, and 5a–f by the reaction of o-haloanilines 1a–g with acrylates 2a–d and isothiocyanates 3aa–3ah/isocyanates 3ba–3bfvia Heck-conjoined amidation/thioamidation and concomitant chemoselective Michael-addition is described. A competitive experiment showed that isothiocyanates were more reactive than isocyanates. The developed methodology offers an efficient chemoselective process for the synthesis of highly functionalized tetrahydroquinazolines under mild and operationally simple reaction conditions from inexpensive and commercially available starting substrates.

 Please wait while we load your content...
Please wait while we load your content...