Design and synthesis of potent hydroxamate inhibitors with increased selectivity within the gelatinase family†
Abstract
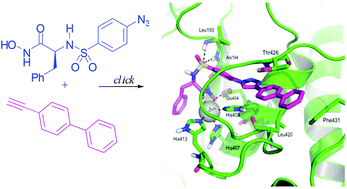
MMP-2 is a validated target for the development of anticancer agents. Herein we describe the synthesis of a new series of potent phenylalanine derived hydroxamates, with increased MMP-2/MMP-9 selectivity compared to analogous hydroxamates described previously. Docking and molecular dynamics experiments have been used to account for this selectivity, and to clarify the role of the triazole ring in the binding process.

 Please wait while we load your content...
Please wait while we load your content...