Rhodium(iii)-catalyzed annulation of arenes with alkynes assisted by an internal oxidizing N–O bond†
Abstract
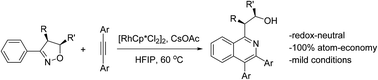
Rh(III)-catalyzed C–H activation of 3-aryl-dihydroisoxazoles in the coupling with diarylacetylenes has been developed under redox-neutral conditions. This reaction occurred under mild conditions with no by-product, and the N–O bond functions as an oxidizing directing group, leading to efficient synthesis of isoquinolines functionalized with a proximal secondary alcohol.

 Please wait while we load your content...
Please wait while we load your content...