Efficient methods for enol phosphate synthesis using carbon-centred magnesium bases†
Abstract
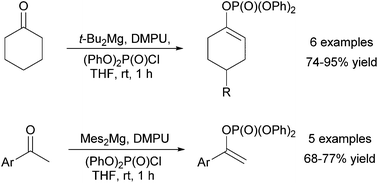
Efficient conversion of ketones into kinetic enol phosphates under mild and accessible conditions has been realised using the developed methods with di-tert-butylmagnesium and bismesitylmagnesium. Optimisation of the quench protocol resulted in high yields of enol phosphates from a range of cyclohexanones and aryl methyl ketones, with tolerance of a range of additional functional units.

 Please wait while we load your content...
Please wait while we load your content...