Arginine analogues incorporating carboxylate bioisosteric functions are micromolar inhibitors of human recombinant DDAH-1†
Abstract
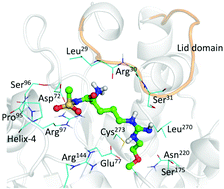
Dimethylarginine dimethylaminohydrolase (DDAH) is a key enzyme involved in the metabolism of asymmetric dimethylarginine (ADMA) and N-monomethyl arginine (NMMA), which are endogenous inhibitors of the nitric oxide synthase (NOS) family of enzymes. Two isoforms of DDAH have been identified in humans, DDAH-1 and DDAH-2. DDAH-1 inhibition represents a promising strategy to limit the overproduction of NO in pathological states without affecting the homeostatic role of this important messenger molecule. Here we describe the design and synthesis of 12 novel DDAH-1 inhibitors and report their derived kinetic parameters, IC50 and Ki. Arginine analogue 10a, characterized by an acylsulfonamide isosteric replacement of the carboxylate, showed a 13-fold greater inhibitory potential relative to the known DDAH-1 inhibitor, L-257. Compound 10a was utilized to study the putative binding interactions of human DDAH-1 inhibition using molecular dynamics simulations. The latter suggests that several stabilizing interactions occur in the DDAH-1 active-site, providing structural insights for the enhanced inhibitory potential demonstrated by in vitro inhibition studies.

 Please wait while we load your content...
Please wait while we load your content...