Synthesis and photophysics of selective functionalized π-conjugated, blue light emitting, highly fluorescent C7-imidazo indolizine derivatives†
Abstract
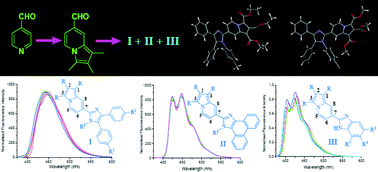
A new family of novel highly fluorescent π-conjugated, C7-imidazole based indolizine derivatives has been prepared in good to excellent yields, catalysed by L-proline in acetonitrile. These are π-conjugated, C7-imidazole based indolizine derivatives covering the emission wavelength range 423–449 nm in acetonitrile with high quantum yields at 25 °C. A thorough photophysical study of all the compounds has been carried out to understand the π-conjugated electronic effect of the imidazole moiety fused at the C7 position on the indolizine motif. In addition, a comparative photophysical study of three selective fluorophores was also carried out in a wide variety of solvents at 25 °C. Finally, the molecular orbitals of the two representative compounds were investigated through DFT calculations to illustrate the pi-conjugate effect.

 Please wait while we load your content...
Please wait while we load your content...