A pyrene-bridged macrocage showing no excimer fluorescence†
Abstract
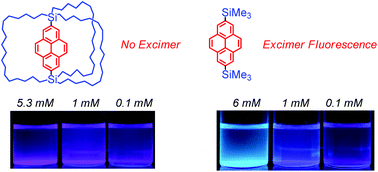
Pyrene is a common organic luminescent material. To improve the fluorescence properties of pyrene, we have designed a pyrene-2,7-diyl bridged macrocage in which the pyrene moiety is sterically protected by the outside alkyl chains. The macrocage shows intense fluorescence from a monomeric excited state without excimer fluorescence even in saturated solutions, although the parent pyrene shows excimer fluorescence in highly concentrated solutions. These results indicate that the steric shielding by the cage prevents the formation of the excimer. Intensities of florescence in the presence of nitrobenzene were investigated to clarify the cage effects on fluorescence quenching. Lower efficiency of the fluorescence quenching caused by intermolecular collision between the caged pyrene (fluorophore) and nitrobenzene (quencher) was revealed by the analysis of the bimolecular quenching constants kq.

 Please wait while we load your content...
Please wait while we load your content...