Importance of topology for glycocluster binding to Pseudomonas aeruginosa and Burkholderia ambifaria bacterial lectins†
Abstract
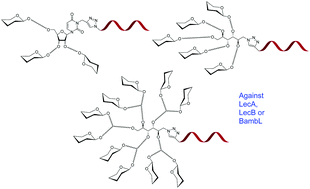
Pseudomonas aeruginosa (PA) and Burkholderia ambifaria (BA) are two opportunistic Gram negative bacteria and major infectious agents involved in lung infection of cystic fibrosis patients. Both bacteria can develop resistance to conventional antibiotherapies. An alternative strategy consists of targeting virulence factors in particular lectins with high affinity ligands such as multivalent glycoclusters. LecA (PA-IL) and LecB (PA-IIL) are two tetravalent lectins from PA that recognise galactose and fucose respectively. BambL lectin from BA is trimeric with 2 binding sites per monomer and is also specific for fucose. These three lectins are potential therapeutic targets in an anti-adhesive anti-bacterial approach. Herein, we report the synthesis of 18 oligonucleotide pentofuranose-centered or mannitol-centered glycoclusters leading to tri-, penta- or decavalent clusters with different topologies. The linker arm length between the core and the carbohydrate epitope was also varied leading to 9 galactoclusters targeting LecA and 9 fucoclusters targeting both LecB and BambL. Their dissociation constants (Kd) were determined using a DNA-based carbohydrate microarray technology. The trivalent xylo-centered galactocluster and the ribo-centered fucocluster exhibited the best affinity for LecA and LecB respectively while the mannitol-centered decafucocluster displayed the best affinity to BambL. These data demonstrated that the topology and nature of linkers were the predominant factors for achieving high affinity rather than valency.
- This article is part of the themed collection: Multivalent Biomolecular Recognition

 Please wait while we load your content...
Please wait while we load your content...