Design of CID-cleavable protein cross-linkers: identical mass modifications for simpler sequence analysis†
Abstract
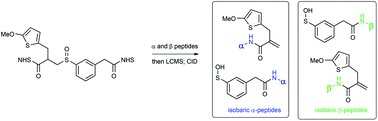
The cross-linking Mass Spectrometry (XL-MS) technique has enormous potential for studying the interactions between proteins, and it can provide detailed structural information about the interaction interfaces in large protein complexes. Such information has been difficult to obtain by conventional structural methods. One of the primary impediments to the wider use of the XL-MS technique is the extreme challenge in sequencing cross-linked peptides because of their complex fragmentation patterns in MS. A recent innovation is the development of MS-cleavable cross-linkers, which allows direct sequencing of component peptides for facile identification. Sulfoxides are an intriguing class of thermally-cleavable compounds that have been shown to fragment selectively during low-energy collisional induced dissociation (CID) analysis. Current CID-cleavable cross-linkers create fragmentation patterns in MS2 of multiple peaks for each cross-linked peptide. Reducing the complexity of the fragmentation pattern in MS2 facilitates subsequent MS3 sequencing of the cross-linked peptides. The first authentic identical mass linker (IML) has now been designed, prepared, and evaluated. Multistage tandem mass spectrometry (MSn) analysis has demonstrated that the IML cross-linked peptides indeed yield one peak per peptide constituent in MS2 as predicted, thus allowing effective and sensitive MS3 analysis for unambiguous identification. Selective fragmentation for IML cross-linked peptides from the 19S proteasome complex was observed, providing a proof-of-concept demonstration for XL-MS studies on protein complexes.

 Please wait while we load your content...
Please wait while we load your content...