Evaluating hydrogen bonding control in the diastereoselective Diels–Alder reactions of 9-(2-aminoethyl)-anthracene derivatives†
Abstract
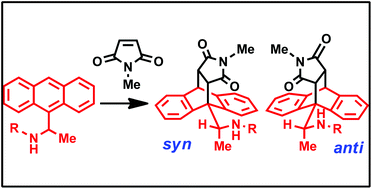
Several 9-(2-aminoethyl)anthracene derivatives were prepared with different nitrogen substitutents including alkyl, acetamide, trifluoroacaeamide and t-butyl carbamate. The selectivity in Diels–Alder cyclodaddition reaction with N-methyl maleimide was evaluated through single crystal X-ray analysis of the products. Models for the change in selectivity with hydrogen bond acceptor are proposed, supported by DFT level calculations.


 Please wait while we load your content...
Please wait while we load your content...