An efficient method for the synthesis of selenium modified nucleosides: its application in the synthesis of Se-adenosyl-l-selenomethionine (SeAM)†
Abstract
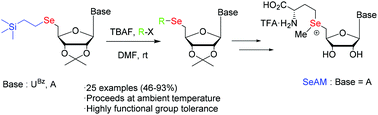
In this paper, we report that a versatile method for the synthesis of 5′-selenium modified nucleosides has been explored on the basis of a 2-(trimethylsilyl)ethyl (TSE) selenyl group as a selenating donor. We demonstrate the broad utility of this method through direct introduction of various functional groups into 5′-TSE-selenonucleosides. This original method offers additional advantages for the preparation of these compounds, such as high functional group tolerance, ready availability of various electrophilic reagents, mild conditions, simple operation, and good yields. The utility of this approach is further demonstrated by the synthesis of Se-adenosyl-L-selenomethionine (SeAM) as a chemical reporter for methyltransferases.

 Please wait while we load your content...
Please wait while we load your content...