Mechanistic insights into the ANRORC-like rearrangement between methylhydrazine and 1,2,4-oxadiazole derivatives†
Abstract
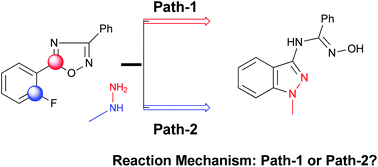
We herein present the first in-depth theoretical study devoted to elucidate the mechanism of the reaction between 1,2,4-oxadiazole derivatives and methylhydrazine. For this purpose, the reaction between methylhydrazine and some polyfluoroaryl-1,2,4-oxadiazoles has been employed as a model reaction. The analysis of the potential energy surface (PES) indicates that the most favorable path involves an initial amine attack at the C(2′) site of the aryl moiety to yield an aryl-hydrazine intermediate whose thermodynamic stability appears as the main determinant of the favored reaction path. Next, the cyclization step leading to a spiro intermediate through a favored 5-exo-trig process appears as the rate determining step. Additionally, this study highlights the relevance of the torsional strain effects on the favored ANRORC pathway. Finally, both the origins of the substituent effects on the regioselectivity patterns as well as the need of using a large excess of nucleophile to afford the favored ANRORC pathway are discussed.

 Please wait while we load your content...
Please wait while we load your content...