Enantioselective cascade double Michael addition of 3-nitro-2H-chromenes and acyclic enones: efficient synthesis of functionalized tricyclic chroman derivatives†
Abstract
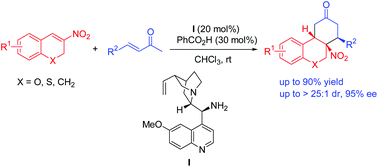
An efficient protocol for the asymmetric construction of enantiomerically enriched tetrahydro-6H-benzo[c]chromenes and their derivatives has been developed. The corresponding products were obtained by the cascade double Michael addition of 3-nitro-2H-chromenes and their derivatives with α,β-unsaturated ketones catalyzed by a combination of a quinine-derived primary amine and benzoic acid. Through this methodology, the desired products could be obtained in moderate to good yields (up to 90%), with excellent diastereoselectivities (up to >25 : 1 dr) and moderate to excellent enantioselectivities (up to 95% ee).

 Please wait while we load your content...
Please wait while we load your content...