Diastereoselective synthesis of pitavastatin calcium via bismuth-catalyzed two-component hemiacetal/oxa-Michael addition reaction†
Abstract
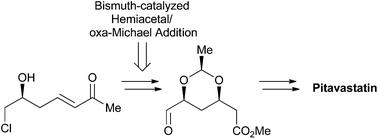
An efficient and concise asymmetric synthesis of pitavastatin calcium (1) starting from commercially available (S)-epichlorohydrin is described. A convergent C1 + C6 route allowed for the assembly of the pitavastatin C7 side chain via a Wittig reaction between phosphonium salt 2 and the enantiomerically pure C6-formyl side chain 3. The 1,3-syn-diol acetal motif in 3 was established with excellent stereo control by a diastereoselective bismuth-promoted two-component hemiacetal/oxa-Michael addition reaction of (S)-α,β-unsaturated ketone 4 with acetaldehyde.

 Please wait while we load your content...
Please wait while we load your content...