Synthesis and evaluation of 2-ethynyl-adenosine-5′-triphosphate as a chemical reporter for protein AMPylation†
Abstract
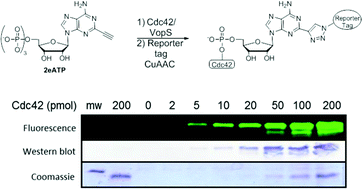
Protein AMPylation is a posttranslational modification (PTM) defined as the transfer of an adenosine monophosphate (AMP) from adenosine triphosphate (ATP) to a hydroxyl side-chain of a protein substrate. One recently reported AMPylator enzyme, Vibrio outer protein S (VopS), plays a role in pathogenesis by AMPylation of Rho GTPases, which disrupts crucial signaling pathways, leading to eventual cell death. Given the resurgent interest in this modification, there is a critical need for chemical tools that better facilitate the study of AMPylation and the enzymes responsible for this modification. Herein we report the synthesis of 2-ethynyl-adenosine-5′-triphosphate (2eATP) and its utilization as a non-radioactive chemical reporter for protein AMPylation.

 Please wait while we load your content...
Please wait while we load your content...