Short and highly efficient synthesis of lipid peroxidation inhibitor pyrrolostatin and some analogues thereof†
Abstract
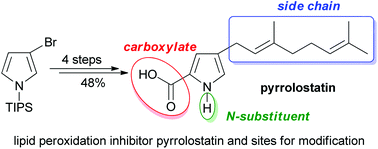
A highly efficient and scalable synthesis of potent lipid peroxidation inhibitor pyrrolostatin is reported (4 steps, 48%). In addition to the synthesis of the natural product, strategies for the preparation of analogues differing in the three structural subunits, the polar head group, the N-substituent and the lipophilic tail are described.

 Please wait while we load your content...
Please wait while we load your content...