Cyclic acetals as cleavable linkers for affinity capture†
Abstract
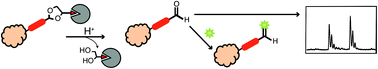
Labeling proteins with biotin is a widely used method to identify target proteins due to biotin's strong binding affinity for streptavidin. Combined with alkyne–azide cycloaddition, which enables the coupling of probes to targeted proteins, biotin tags linked to an alkyne or azide have become a powerful tool for purification and analysis of proteins in proteomics. However, biotin requires harsh elution conditions to release the captured protein from the bead matrix. Use of these conditions reduces signal to noise and complicates the analysis. To improve affinity capture, cleavable linkers have been introduced. Here, we demonstrate the use of a cyclic acetal biotin probe that is prepared easily from commercially available starting materials, is stable to cell lysates, yet is cleaved under mildly acidic conditions, and which provides an aldehyde for further elaboration of the protein, if desired.

 Please wait while we load your content...
Please wait while we load your content...