Natural nitric oxide (NO) inhibitors from the rhizomes of Curcuma phaeocaulis†
Abstract
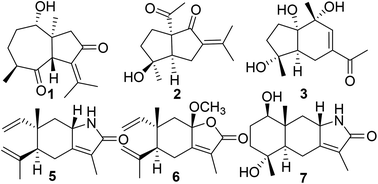
An exploration we carried out for isolating nitric oxide (NO) inhibitors from the rhizomes of Curcuma phaeocaulis afforded one new salvialane-type sesquiterpene, phasalvione (1), two novel nor-sesquiterpenes, phaeocaudione (2) and phaeocauone (3), one aromatic acid 3-methyl-4-(3-oxo-butyl)-benzoic acid (4), two γ-elemene-type sesquiterpenes, 8β(H)-elema-1,3,7(11)-trien-8,12-lactam (5) and 8β-methoxy-isogermafurenolide (6), one eudesmane-type sesquiterpene, phaeusmane I (7), and one cyclic diarylheptanoid, phaeoheptanoxide (8). Their structures were established based on extensive spectroscopic analysis. The absolute configurations of compounds 1 and 2 were assigned using the circular dichroism data of the [Rh2(OCOCF3)4] complex, and the absolute configuration of 1 was further established by single crystal X-ray crystallography. It is noteworthy that compounds 5–7 were racemates analyzed by chiral HPLC. Furthermore, the inhibitory effects of the isolated compounds on nitric oxide production in LPS-activated macrophages were evaluated. Compounds 1, 3 and 4 showed strong inhibitory activities on NO production with IC50 values of 7.46 ± 0.69, 2.35 ± 0.17 and 3.49 ± 0.31 μM, respectively. A plausible biosynthetic pathway for 1–4 in C. phaeocaulis was also discussed.

 Please wait while we load your content...
Please wait while we load your content...